In this animated lecture, I will teach you the concept of mixture, different types of mixture, homogeneous mixture, heterogeneous mixture, difference between The key difference between homogeneous and heterogeneous is that homogeneous materials and mixtures have the same uniform composition and properties throughout whereas heterogeneous materials and mixtures do not have either uniform composition or uniform properties Homogeneous and heterogeneous are two different wordsDifference between compound and homogeneous mixture learning objectives explain the difference between a pure substance and a mixture explains the difference between an element and a compound explains the difference between a homogeneous mixture and a heterogeneous mixture a useful way to organize our understanding of matter is to think of a

Difference Between Homogeneous Mixture And Heterogeneous Mixture
Difference between homogeneous and heterogeneous mixtures with examples
Difference between homogeneous and heterogeneous mixtures with examples-What is the difference between heterogeneous and homogeneous mixture Define the mixture Define homogeneous mixture Date examples of homogeneous mixtures How do you like coffee?Homogeneous Mixture Definition A homogeneous mixture is a mixture of substances blended so thoroughly that you cannot see individual substances Every sample of the mixture will show the same amounts of each substance Homogeneous mixtures can be solid, liquid, gas, or plasma mixtures



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples
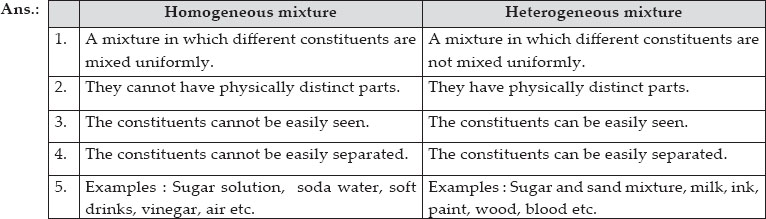
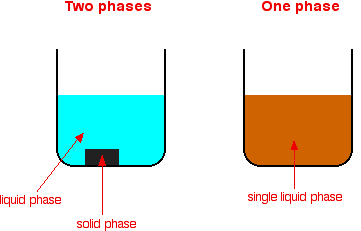
Homogeneous mixture Heterogeneous mixture It can't be separated out physically It can be separated out physically 'homo' means the same 'hetero' means different Example a mixture of alcohol and water Example a mixture of sodium chloride and sand Difference between homogeneous and heterogeneousBy definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers What is difference between homogeneous mixture and heterogeneous
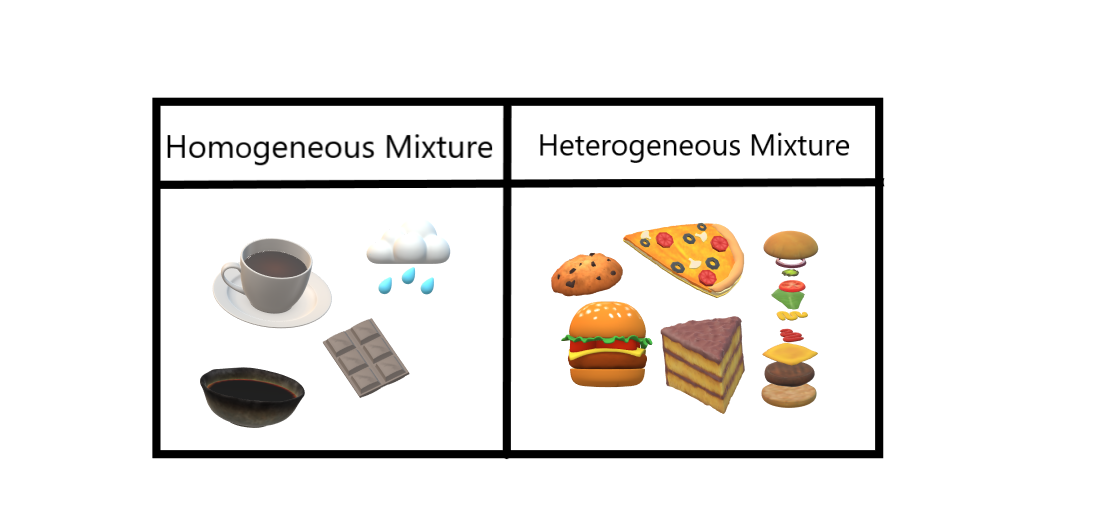
This video is in simple language about Difference between homogenous and heterogeneous mixturesClass 9Chapter 2Is Matter Around Us PureThe difference between heterogeneous and homogeneous mixtures is the degree to which materials are mixed together and the uniformity of their composition A Homogeneous mixture is a mixture in which components that make up mixture are uniformly distributed throughout the mixture The composition of the mixture is same throughout Examples of Homogeneous Mixture are tea, rain, soya sauce, chocolate, and Heterogeneous Mixture are cookies, pizza, burger layering, cake, etc Difference between Homogeneous and Heterogeneous Mixtures
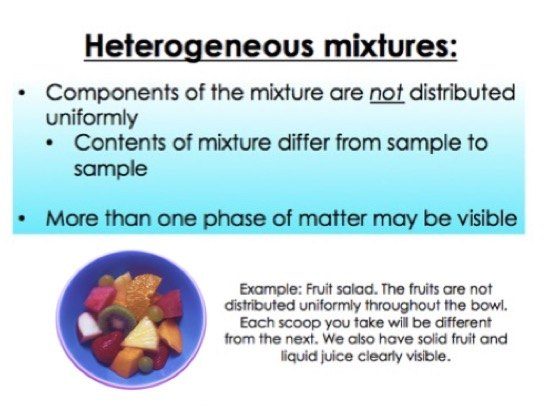
Heterogeneous mixture (I) Homogeneous mixtures have uniform composition throughout the mixture (II) The whole mixture is in same phase (III) Components are not visible to the naked eye (IV) Components cannot be separated easily Eg Sugar Water → Sugar solution (I) Heterogeneous mixture have composition which may vary from point to pointMany people enjoy a cup of coffee at some point during the day Some can drink black, while others can put cream (or some milk substitute) and sugar in their coffee In many cases, the mixed elements can be easily separated again For example, a salad Unlike homogeneous mixtures, in heterogeneous mixtures it is very easy to identify, even with the naked eye, what are the different components that make them up This makes it much easier to separate these mixes at the same time For example water and oil / water and sand Examples of Heterogeneous Mixtures




Differentiate Between Homogeneous And Heterogeneous Mixtures With Examples Youtube
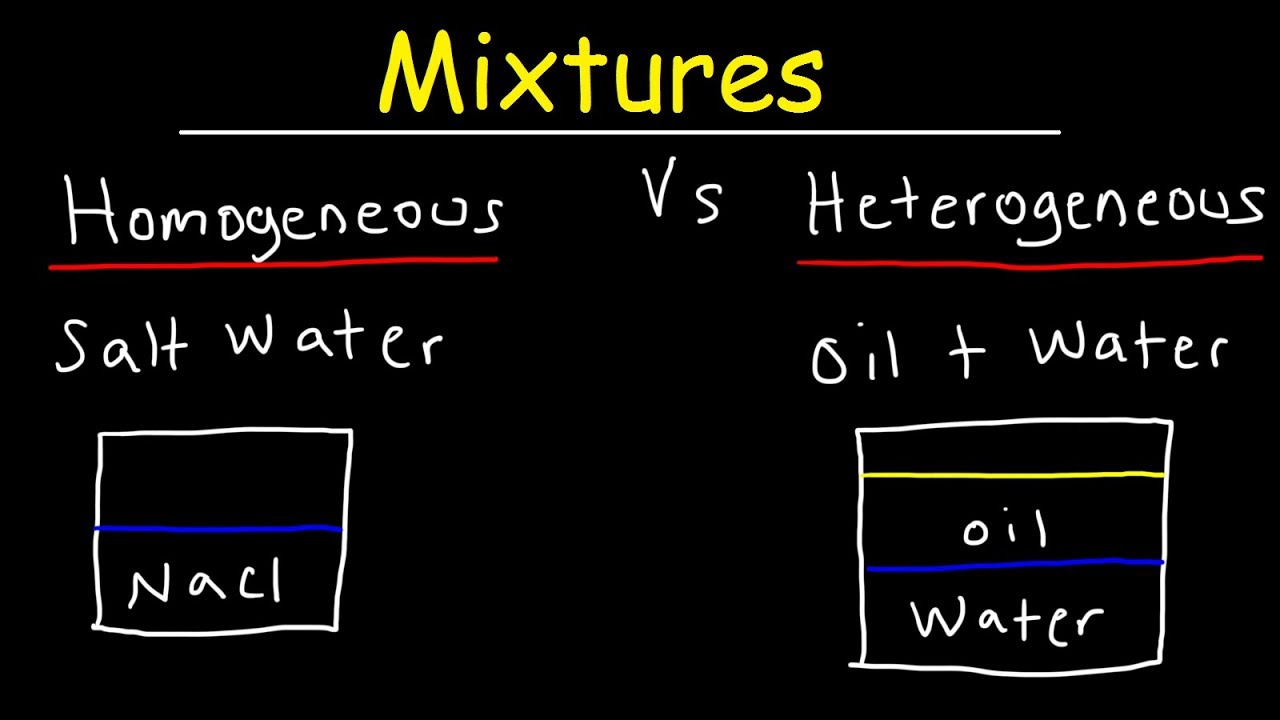



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube
Examples of heterogeneous mixtures include sand, oil and water, and chicken noodle soup What is homogeneity and heterogeneity? A homogeneous mixture is a mixture having a uniform composition throughout the mixture For example salt in water, sugar in water, copper sulphate in water A heterogeneous mixture is a mixture having a nonuniform composition throughout the mixture For example sodium chloride and iron fillings, salt and sulphur, oil and water Question Difference between homogeneous and heterogeneous mixture with examples Answer Homogeneous Mixture A mixture in which its different constituents are mixed uniformly (uniform composition);




Difference Between Homogeneous Mixture And Heterogeneous Mixture




List The Point Of Difference Between Homogeneous And Heterogeneous Mixture Science Is Matter Around Us Pure Meritnation Com
Start studying Heterogeneous vs Homogeneous Mixtures Learn vocabulary, terms, and more with flashcards, games, and other study tools Pure substances in a heterogeneous mixture can be easily separated without using such processes Phase of matter The homogeneous mixture is only in one phase of matter The heterogeneous mixture is always in two or more than two different phases of matter Examples When we mix alcohol in water, it exists in a uniform physical stateThe terms homogeneity and heterogeneity are used to describe the uniformity and regularity in spatial distribution of geomaterial properties in



Difference Between Homogeneous And Heterogeneous Welding



Http Punainternationalschool Com Assets Upload Ck Images Class ix chemisty july aug material Pdf
Example 1 Mixture of Oil and Water is a heterogeneous Mixture We are able to see oil and water clearly separately in the mixture Example 2 Mixture of Salt (Sodium Chloride) and Iron filings is a heterogeneous mixture The particles of salt and Iron filings can be seen and distinguished easily Difference between Homogeneous and Heterogeneous mixturesHomogeneous mixtureHeterogeneous mixture1) These are called as solutionsThese are called as suspensions/colloids2) Substances are Uniformly distributedThese substances are Unevenly distributed3) These are not visible to the naked eye, but visible through the microscopeThese are easily visible to the naked eye and also through microscope4) The particles appear smaller inThe difference between heterogeneous and homogeneous mixtures is the degree to which materials are mixed together and the uniformity of their composition A homogeneous mixture is a mixture in which components that make up mixture are uniformly distributed throughout the mixture The composition of the mixture is same throughout




Difference Between Homogeneous And Heterogeneous Reactions Compare The Difference Between Similar Terms




Classification Of Matter Chemistrygod
Heterogeneous mixtures are not uniform in composition Examples would be wood or bloodBasically we subdivide a mixture in homogeneous and heterogeneous mixtures In simple words, in a homogeneous mixture, it is not possible to easily differentiate between its components The components in a heterogeneous mixture can be easily differentiated An example of a homogeneous solution can be a salt solution6 rows Homogeneous mixture Heterogeneous mixture It has a uniform composition It has a




Differentiate Between Homogeneous And Heterogeneous Mixtures With Suitable Examples Swiflearn




Compound Vs Mixture Difference And Comparison Diffen
Difference between Homogeneous and Heterogeneous Mixture Homogeneous mixture Heterogeneous mixture It can't be separated out physically It can be separated out physically 'homo' means the same 'hetero' means different Example a mixture of alcohol and water Example a mixture of sodium chloride and sandDespite being a part of the mixture The mixtures are divided into two main categories known as homogeneous mixtures and heterogeneous mixtures The Homo and Straight terms represent the most important difference between homogeneous and heterogeneous mixtures The Homo prefix refers to uniformity while straight indicates nonuniformity Heterogeneous mixtures are either colloids or suspensions, depending on the size of the dispersed particles Read Also Compound vs Mixture Definition, 12 Major Differences, Examples 8 Major Differences (Homogenous vs Heterogeneous Mixture)



1




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
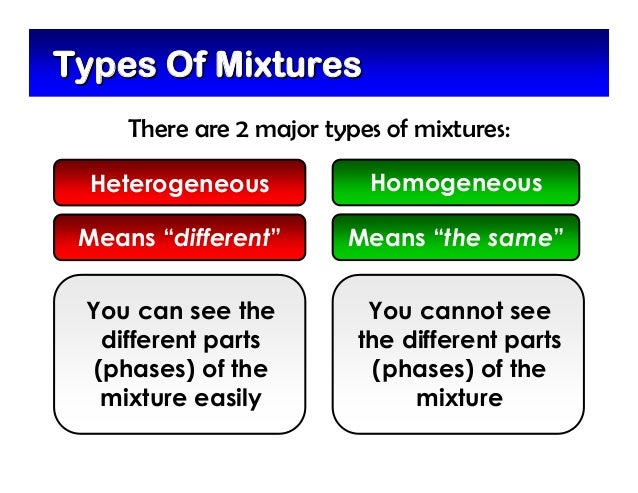
Homogeneous and heterogeneous are two types of mixtures that are studied and apart of science To start identifying the difference between these two mixtures, we can look at the prefixes of each word The prefix homo means single In relation to a homogeneous mixture, this means the mixture only goes through one phaseA heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture The composition varies from one region to another with at least two phases that remain separated from each other, with clearly identifiable properties homogeneous vs heterogeneous The difference between homogeneous mixtures and heterogeneous mixtures is a matter of scale the heterogeneous mixture can be seen on beaches where sand included many particles like coral, shells and organic matter, etc they all can be separated easily hence known as a heterogeneous mixture but when we take a large amount of sand, it's impossible to separate all the matter, which terns as a homogeneous mixture example
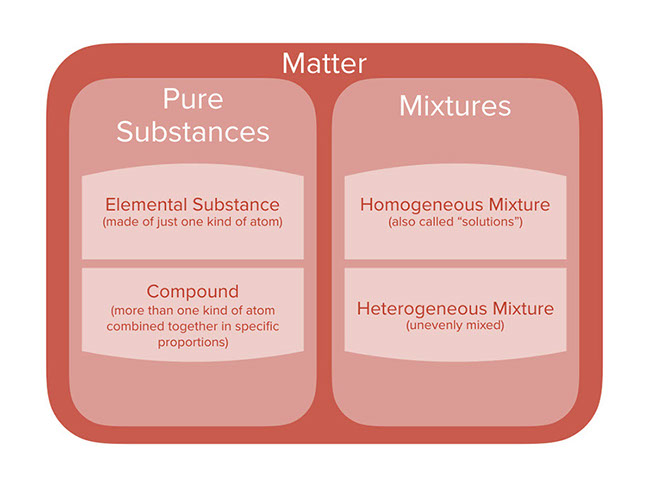



Lesson Categories Of Chemicals And Mixtures



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples
A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers With that being said, what is the difference between a homogeneous mixture and a heterogeneous mixture quizlet? Types and examples of homogeneous mixtures Liquid mixture Pure water, vinegar, coconut oil, etc Gas Mixtures Air in the Atmosphere Solid mixtures For example mineral ores, alloys such as steel, bronze, brass What is a Heterogeneous mixture?Examples of homogeneous mixture A glass of lemonade (mixture of water, lemon juice, sugar, salt) is a homogeneous mixture because the dissolved sugar, salt, and lemon juice are evenly distributed throughout the entire sample You can't easily separate the lemon juice from the water;




Difference Between Homogeneous And Heterogeneous Mixtures Homogeneous Vs Heterogeneous Youtube



Ppt Objective I Will Distinguish Between Homogeneous And Heterogeneous Mixtures Powerpoint Presentation Id
Examples Homogeneous Mixture Some examples of homogeneous mixtures are most alloys, seawater, brass, vinegar, air, blood, natural gas, etc Heterogeneous Mixture Salt and pepper together make a heterogeneous mixture Other examples include soda, concrete, sand and sugar, muddy water, chocolate chip cookies, all kinds of suspensions, etc Conclusion6 rows In homogeneous mixtures, there seems to be only one component (solute and solvent), but inDifference between Homogenous and Heterogeneous Mixture Chemistry is one of the critical subjects that deal with different terms like mixtures, compounds, elements, etc Chemistry is the subject used for various research experiments



Sxxv4y Ycx9hem




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms
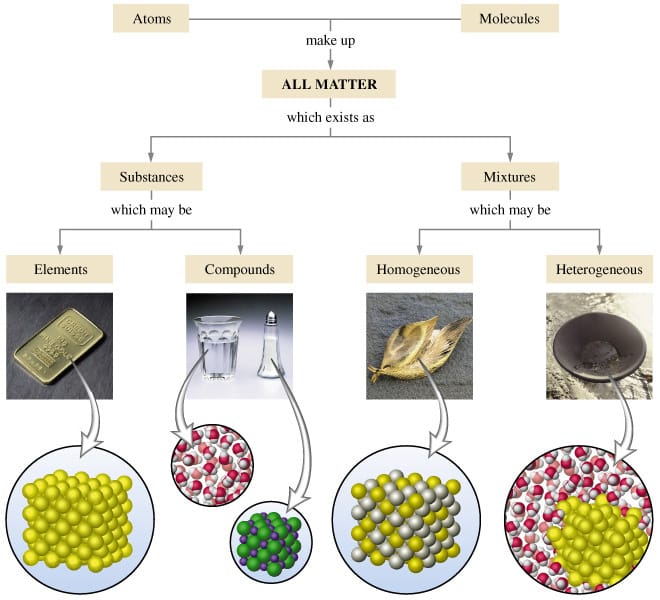
Mixtures are also called impure substances because it is composed of different kinds of components Mixtures are further divided into two categories which are a homogeneous mixture and heterogeneous mixture Example; To identify the nature of a mixture, consider its sample size If you can see more than one phase of matter or different regions in the sample, it is heterogeneous If the composition of the mixture appears uniform no matter where you sample it, the mixture is homogeneous The difference between heterogeneous and homogeneous mixtures is the degree at which the materials are mixed together and the uniformity of their composition A homogeneous mixture is a mixture where the components that make up the mixture are uniformly distributed throughout the mixture



Difference Between Homogenous And Heterogeneous Mixture Javatpoint




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube
Differentiate between Homogeneous and Heterogeneous mixture with examples by Jaishree Gorane Leave a Comment List the points of difference between Homogeneous and Heterogeneous mixtures Other examples of homogeneous mixtures Air, water and alcohol, water and sugar HETEROGENEOUS It refers to combinations that are not totally uniform and in many cases are clearly visible when "mixed"Heterogeneous mixtures are composed of two or more substances that exhibit specific characteristics




Chemistry For Kids Chemical Mixtures
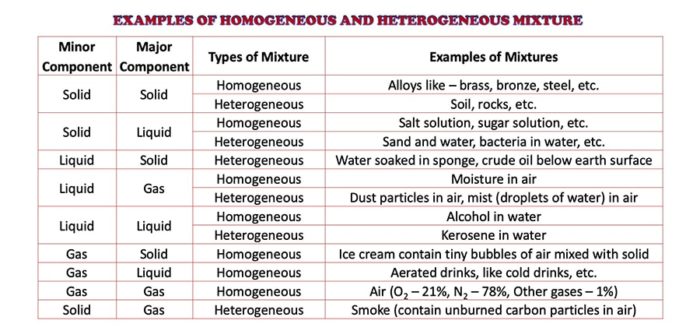



Homogeneous Heterogeneous Mixture Definition Examples Selftution
5 rows The components of homogeneous mixtures are not physically distinct A heterogeneous mixtureCorrect answers 2 question ⚠⚠need ⚠⚠ difference between homogeneous and heterogeneous mixture ⬅⬅ full explanation with examples don't spam The homogeneous mixture is only in the one phase of matter The heterogeneous mixture is always in two or more than two different phases of matter Examples When we mix alcohol in water, it exists in the uniform physical state



Mixture
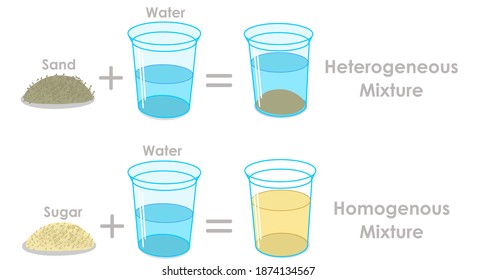



Mixtures Images Stock Photos Vectors Shutterstock
It has no visible boundaries of separation betweenWater and sand, salt and water Main Differences Between Homogeneous and Heterogeneous Mixtures Homogeneous Mixture isn't really visible to the human eye but can be seen under a magnification lens Whereas Homogeneous Mixtures are commonly known as solutions (since the solute and solvent are mixed thoroughly) On the other




1 Differentiate Between Homogon Eous Aid Heterogeneous Mixt Scholr
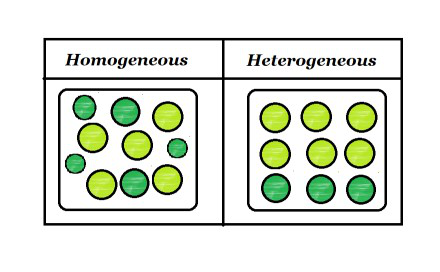



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Difference Between Homogeneous And Heterogeneous Equilibrium Compare The Difference Between Similar Terms
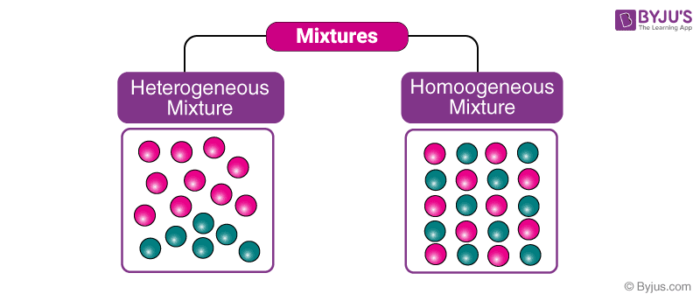



Heterogeneous And Homogeneous Mixture Differences Videos Examples




List 5 Difference Between Homogenous Anf Heterogenous Mixture Brainly In




Homogenous Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture




Examples Of Heterogeneous Mixtures Types Made Simple




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards




Heterogeneous And Homogeneous Mixture Differences Videos Examples




What Is A Heterogeneous Mixture Definition And Examples



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Classifying Matter Schoolworkhelper



Homogeneous And Heterogeneous Mixtures Youtube




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com




Q 9 List The Points Of Differences Between Homogeneous And Heterogeneous Mixtures Brainly In




Homogenous Definition And Examples Biology Online Dictionary



Q Tbn And9gctbkedxyjwahe7d2u37wpo4weq 7udcstrph24u4t30kpkayj6h Usqp Cau




Pure Substances And Mixtures Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture



Homogeneous And Heterogeneous Mixtures Geeksforgeeks




Homogeneous Vs Heterogeneous Mixtures Difference And Comparison Diffen




What Is The Difference Between Homogeneous And Heterogeneous Mixture




Q2 Differentiate Between Homog Lido




Yr4cxrz9i5bjsm



What Does It Mean When A Mixture Is Heterogeneous



What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com




Oxford Investigation




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




Homogeneous Mixture Example Food




Mixture Wikiwand




Solutions And Mixtures Flashcards Quizlet



Types Of Catalysis



Homogenous Vs Heterogeneous Mixture Definition 8 Key Differences Examples



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




10 Examples Of Mixtures




Write 5 Difference Between Homogeneous And Heterogeneous Science Is Matter Around Us Pure Meritnation Com




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo




Heterogeneous And Homogeneous Mixture Differences Videos Examples



Some Basic Definitions Introductory Chemistry



Homogeneous And Heterogeneous Mixtures




Mixtures And Solutions Cpd Rsc Education




Homogeneous Mixture Examples In Daily Life




2nd Is Matter Around Us Pure Science




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Interesting Chemistry Difference Between Homogeneous And Heterogeneous Mixture Chemical Chemistrynotes Chemistry Facebook




Write The Difference Between Homogeneous And Heterogeneous Solution With An Example Brainly In




Difference Between Homogenous And Heterogenous Mixtures Youtube




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous




Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture Venn Diagram Examples Venn Diagram Template
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures



How Can Heterogeneous Catalysts Differ From Homogeneous Catalysts Quora




Homogeneous Mixture Examples In Kitchen




Homogeneous Mixture Definition Examples Tutors Com
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Heterogeneous And Homogeneous Mixtures With Examples Study Guide Brighthub Education




Homogeneous Mixture Example




Homogeneous Mixture Definition Examples Tutors Com




Difference Between H Mixture And Heterogeneous Mixture Brainly In



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Vihonbtm8o0zpm



3




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



1



Mixture
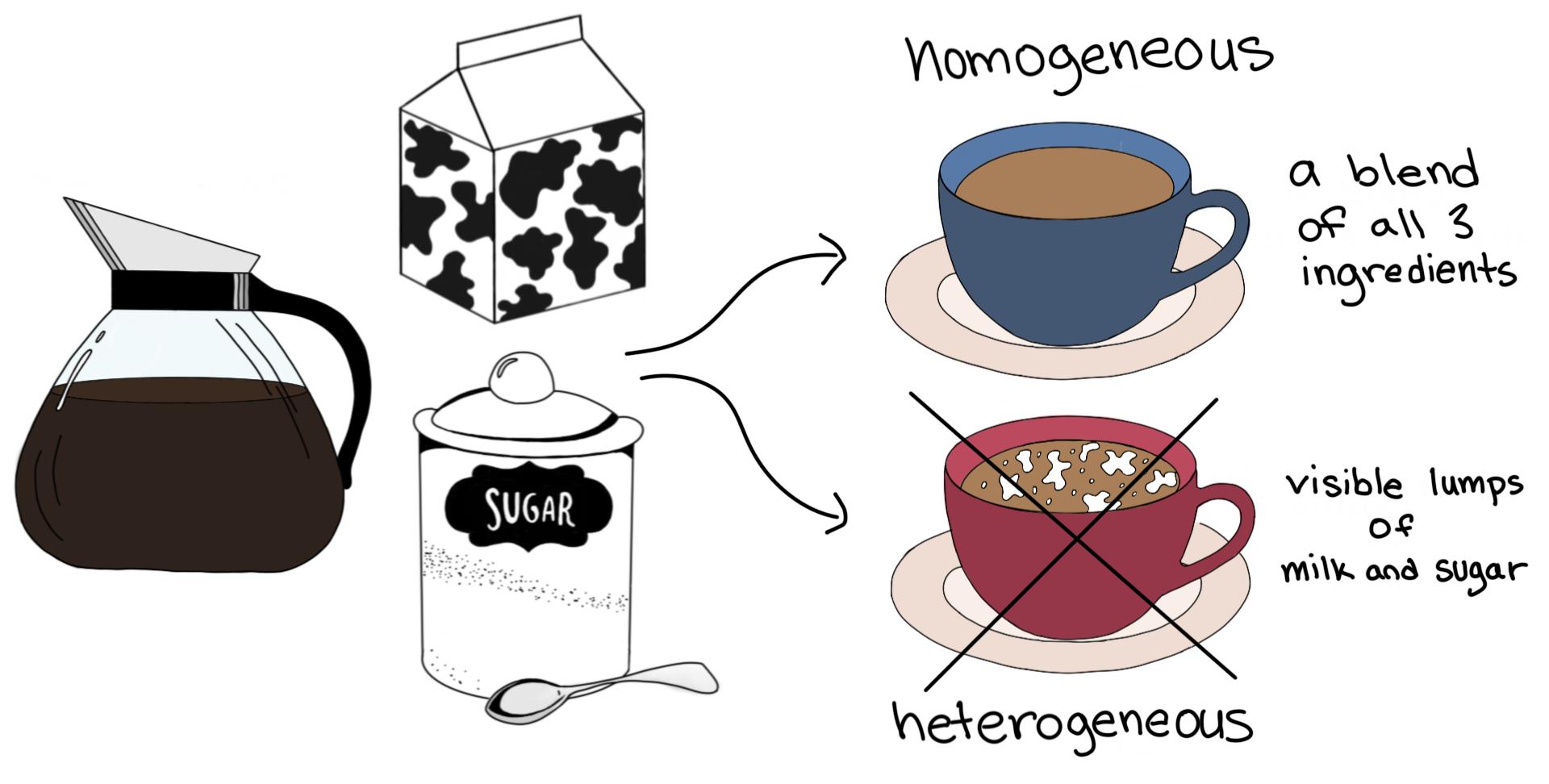



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




What Is Difference Between Heterogeneous And Homogeneous Brainly In




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms




Homogeneous Mixture Definition Examples Tutors Com




List The Points Of Differences Between Homogeneous And Heterogeneous Mixtures




What Is A Homogeneous Mixture Definition And Examples




List The Points Of Difference Between Homogeneous And Heterogeneous Mixtures Brainly In
:max_bytes(150000):strip_icc()/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples
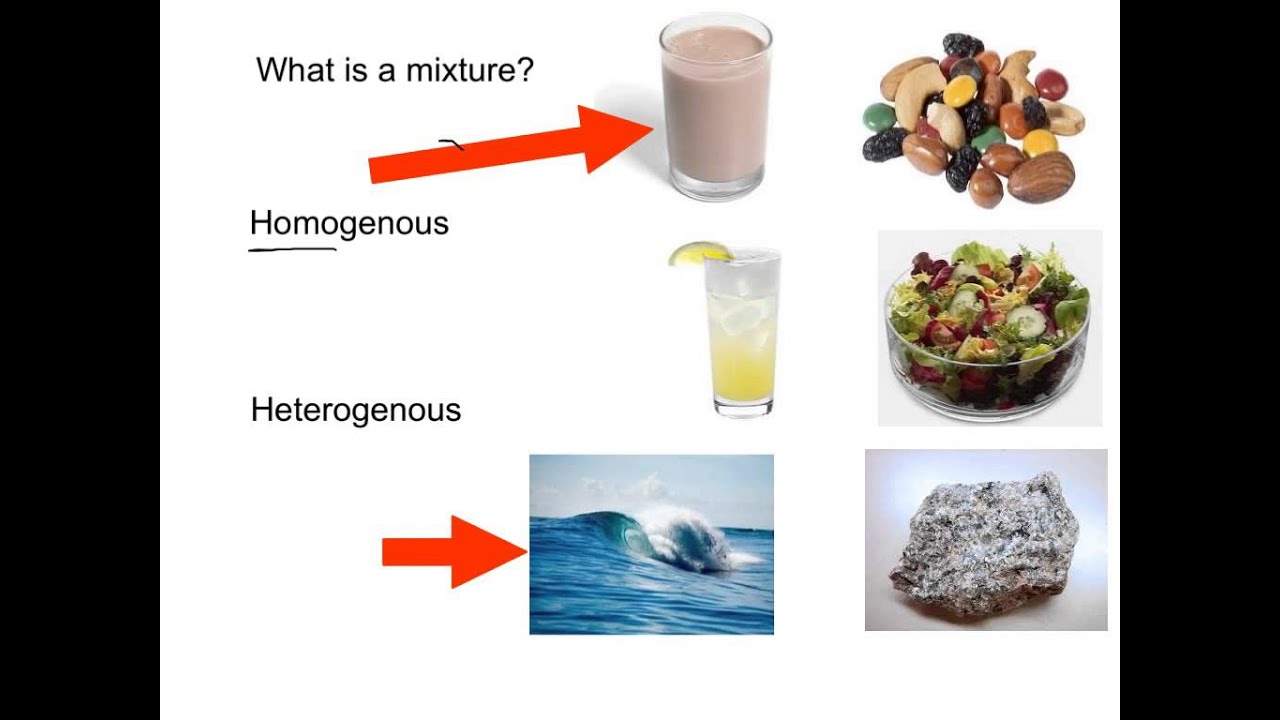



Mixtures Youtube




List The Point Of Difference Between Homogenous And Heterogenous Mixtures
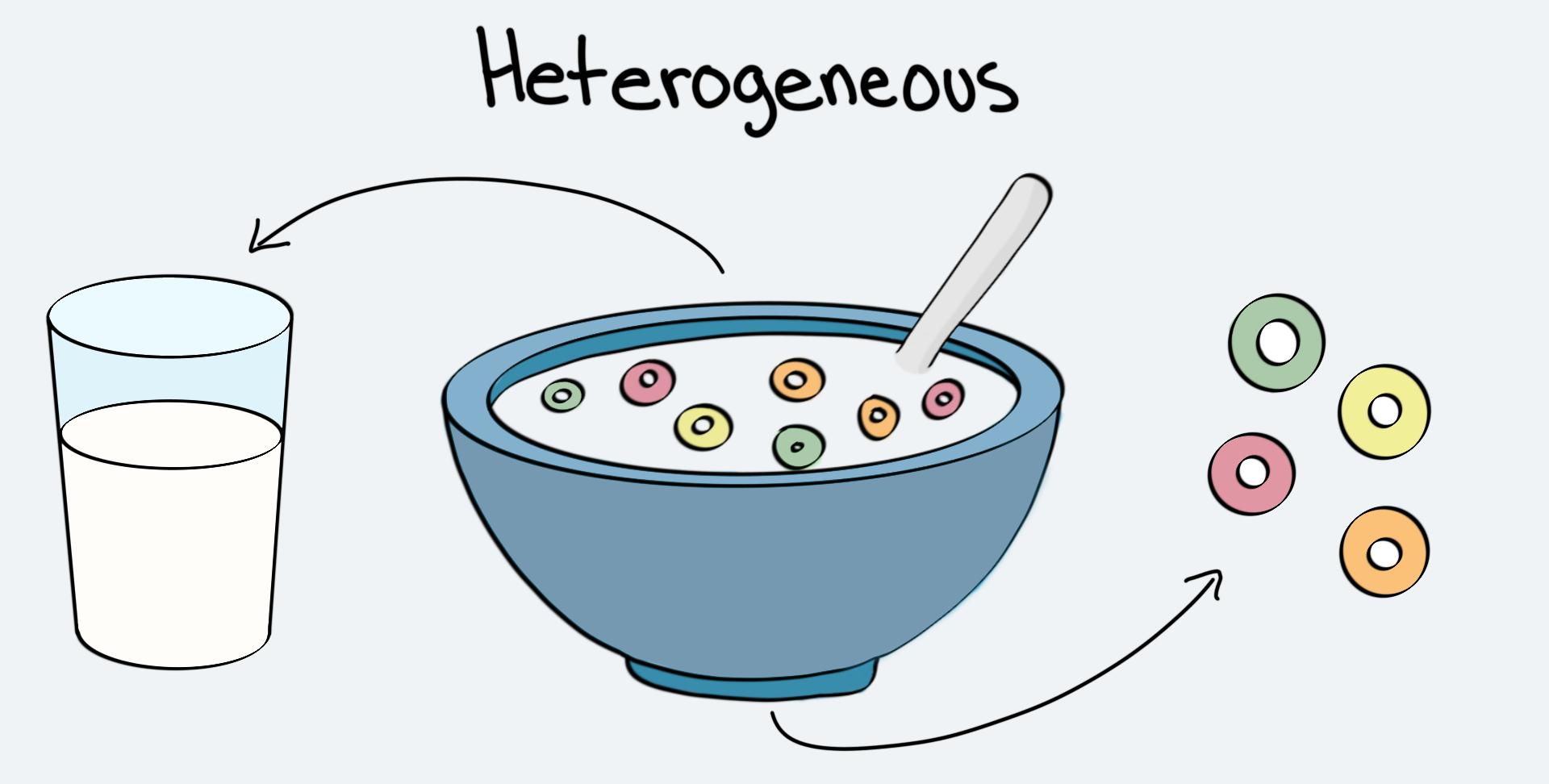



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




What Is The Difference Between Homogeneous And Heterogeneous Mixtures




Difference Between Homogenous And Heterogenous Mixture



Http Punainternationalschool Com Assets Upload Ck Images Class ix chemisty july aug material Pdf




3 4 Classifying Matter According To Its Composition Chemistry Libretexts




What Is The Difference Between Heterogeneous Mixture Vs Homogenous Mixture Brainly Com



0 件のコメント:
コメントを投稿